Navigation
News
-
Lithium-6 or not?
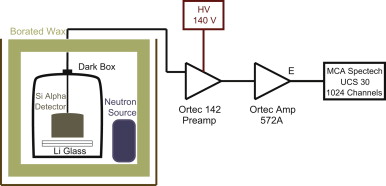
Lithium-6 is a stable isotope of lithium that captures neutrons, splitting apart into an alpha and a triton. If you put regular scintillating glass on top of glass containing Li-6, it makes a very efficient ultracold neutron detector. The trouble is, once they're bonded together, can you tell which side contains the Li-6? Blair Jamieson and Lori Rebenitsch figured out a way that doesn't require an ultracold neutron source!
They used a neutron-emitting radioactive source, and placed a silicon detector very close to the surface of the glass. If the glass emitted alpha particles or tritons, the silicon detector would be able to detect them thereby indicating that the Li-6 side was "up". This offered a useful cross-check that the correct side of the glass would be seen by the ultracold neutrons, once the glass is glued to a light-sensitive detector in the real experiment.
Read the article in Nuclear Instrumentation in Physics Research APosted on 01 Jun 2015Powered by CuteNews


